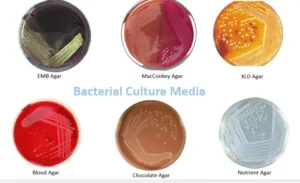
What is media?
Media, also called culture media or growth media, is a liquid or gel that supports the growth of microorganisms
Types of Media preparation
- Blood agar media
- Chocolate agar media
- CLED agar media
- Muller-Hinton media
- MacConkey agar media
- Simmons citrate agar media
- Triple Sugar Iron (TSI) agar media
1: Blood agar media
Principle
Blood agar is a general-purpose, enriched medium that is frequently used to cultivate bacteria that require nutrients and to distinguish between various bacteria according to their hemolytic characteristics.
Composition of blood agar
- 5% peptone
- 3% beef extract/ yeast extract
- 5% NaCl
- 5% sheep blood
- 5% agar
Materials
- Blood agar base
- Weighing balance
- Petri plates
- Filter paper
- Distilled water
- Cotton plug
- Aluminum foil
- Indicator tape
- Hot plate
- Autoclave
Procedure
- Filter paper was placed on a Petri plate, which was then placed on a weighing scale and set to zero.
- 40 grams of blood agar foundation were weighed using a weighing balance.
- In a conical flask, suspend 40g of blood agar base in 1000ml of distilled water. Place a cotton plug on the flask, cover it with aluminum foil paper, and secure it with indicator tape.
- The mixture should now be heated on a hot plate to eliminate any clumps.
- Next, autoclave the dissolved mixture for 15 minutes at 15 pounds of pressure and 121°C.
- The medium should be allowed to cool to room temperature after autoclaving.
- Add 50ml of warm sheep or goat blood to the agar, which should be between 50 and 60°C, and mix gently.
- After the temperature reaches 35 to 40°C, the Petri plates are filled with medium.
- In order to evaporate the moisture, the plates were partially covered with a cap.
- It is recommended that solidified medium plates be incubated for 24 hours before being refrigerated.
-
Chocolate Agar Media
Principle
Blood agar is heated to create chocolate agar, which breaks down red blood cells (RBCs) and provides nutrients that support the development of fastidious bacteria, particularly Neisseria and Haemophilus species. Its name comes from the fact that RBC lysis produces a medium chocolate brown hue.
Composition of Chocolate Agar
- 5% peptone
- 3% Beef extract/ Yeast extract
- 5% Chocolate Agar base
- 5% NaCl
- 5% sheep blood
Materials
- Weighing balance
- Conical flask
- Measuring cylinder
- Filter paper
- Petri dish
- Aluminum foil paper
- Cotton plug
- Indicator tape
- Distilled water
- Hot plate
- Autoclave
Procedure
- Placed filter paper on a Petri dish, set the weighing balance to zero, and left it there.
- Using a weighing balance, 40 grams of blood agar foundation were weighed.
- Fill a conical flask with 40g of blood agar base and 1000ml of distilled water. Cover the
- Flask with aluminum foils paper, secure it with indicator tape, and place a cotton plug on it.
- To get rid of the clumps, heat the mixture on a hot plate.
- The dissolved mixture is then autoclaved for 15 minutes at 121°C and 15 pounds of pressure.
- The medium should be allowed to cool to room temperature after autoclaving.
- Add 50ml of warm sheep or goat blood to the agar, which should be between 50 and 60°C, and mix gently.
- The mixture is then heated once more on a hot plate.
- After the temperature reaches 35 to 40°C, the Petri plates are filled with medium.
- In order to evaporate the moisture, the plates were partially covered with a cap.
- It is recommended that solidified medium plates be incubated for 24 hours before being refrigerated.
-
Cysteine-Lactose-Electrolyte-Deficient (CLED) Agar Media
Principle
Cysteine-Lactose-Electrolyte-Deficient (CLED) agar is a non-selective medium that may sustain the development of the majority of urine pathogens and is often employed for routine diagnostic urinary bacteriology. It encourages the development of every potential urine pathogen. Additionally, the medium offers unique colony morphology. However, due to its low electrolyte levels, it prevents the spread of proteus species.
Composition of CLED agar Media
- Peptone (4 g/l)
- Beef extract / Yeast extract (3g/l)
- Lactose (10g/l)
- L-Cystine (0.128g/l)
- Bromothymol Blue (0.02g/l)
- NaCl (0.5%)
- Agar (15g/l)
Materials
- Weighing balance
- Conical flask
- Measuring cylinder
- Filter paper
- Petri dish
- Aluminum foil paper
- Cotton plug
- Indicator tape
- Distilled water
- Hot plate
- Autoclave
Procedure
- A Petri plate was used, filter paper was placed on it, and the weighing balance was held at zero.
- A weighing balance was used to weigh 36 grams of CLED Agar Base.
- Using a conical flask, suspend 36g of CLED agar base in 1000ml of distilled water.
- Cover the flask with aluminum foil paper, secure it with an indicator taper, and place a cotton plug on it.
- In order to eliminate the clumps, cook the mixture on a hot plate.
- After the mixture has dissolved, autoclave it for 15 minutes at 121°C and 15 pounds of pressure.
- The medium should be allowed to cool to room temperature after autoclaving.
- Fill the Petri plates with the medium once the temperature reaches 35 to 40°C.
- To help the moisture evaporate, partially cover the plates with a cap.
- It is recommended that solidified medium plates be incubated for 24 hours before being refrigerated.
-
MacConkey Agar Media
Principle
MacConkey agar is a selective and differential medium used to isolate and differentiate non-fastidious gram-negative rods, especially those belonging to the genus Pseudomonas and the family Enterobacteriaceae
Composition of MacConkey Agar Media
- Bile salts (1.5g/l)
- Lactose (10g/l)
- NaCl (5g/l)
- Peptone (17g/l)
- Protease peptone (3g/l)
- Neutral red (0.03g/l)
- Crystal violet (0.001g/l)
- Agar (13.5g/l)
- Distilled water (1ltr)
Materials
- Weighing balance
- Conical flask
- Measuring cylinder
- Filter paper
- Petri dish
- Aluminum foil paper
- Cotton plug
- Indicator tape
- Distilled water
- Hot plate
- Autoclave
Procedure
- Filter paper was placed on a Petri plate, which was then placed on a weighing scale and set to zero.
- 51 grams of MacConkey Agar Base were weighed using a weighing balance.
- In a conical flask, suspend 51g of MacConkey agar base in 1000ml of distilled water.
- Place a cotton plug on the flask, cover it with aluminum foil paper, and secure it with an indicator taper.
- The mixture should now be heated on a hot plate to eliminate any clumps.
- Next, autoclave the dissolved mixture for 15 minutes at 15 pounds of pressure and 121°C.
- The medium should be allowed to cool to room temperature after autoclaving.
- Fill the Petri plates with the medium once the temperature reaches 35 to 40°C.
- To help the moisture evaporate, partially cover the plates with a cap.
- It is recommended that solidified medium plates be incubated for 24 hours before being refrigerated.
-
Mueller Hinton (MH) agar
Principle
Hinton Muller Agar and casein starch beef extract hydrolyzed with beef extract acid make up agar medium. Carbon, nitrogen, vitamins, amino acids, sulfur, and other vital nutrients are provided by beef extract and casein acid hydrolysate. Energy-producing dextrose is created when starch is hydrolyzed. The primary substance that forms solids is agar. Tetracycline and sulfonamide inhibitor concentrations, including those of calcium ions and thymine, are regulated to promote healthy development and avoid interfering with susceptibility tests.
Composition of Mueller Hinton (MH) agar
- Starch (1.5g/l)
- Beef extract / yeast extract (2g/l)
- NaCl (0.5%)
- Agar (12g/l)
Materials
- Weighing balance
- Conical flask
- Measuring cylinder
- Filter paper
- Petri dish
- Aluminum foil paper
- Cotton plug
- Indicator tape
- Distilled water
- Hot plate
- Autoclave
Procedure
- Filter paper was placed on a Petri plate, which was then placed on a weighing scale and set to zero.
- Mueller Hinton Agar Base (38 grams) was weighed using a weighing balance.
- Place 38g of Mueller Hinton agar foundation in a conical flask with 1000ml of distilled water, cover the flask with aluminum foil paper, tighten the indicator taper, and place a cotton plug on it.
- The mixture should now be heated on a hot plate to eliminate any clumps.
- Next, autoclave the dissolved mixture for 15 minutes at 15 pounds of pressure and 121°C.
- The medium should be allowed to cool to room temperature after autoclaving.
- Fill the Petri plates with the medium once the temperature reaches 35 to 40°C.
- In order to evaporate the moisture, the plates were partially covered with a cap.
- It is recommended that solidified medium plates be incubated for 24 hours before being refrigerated.
- Simmons citrate (SIM) agar
Principle
The medium Simmons citrate agar is both differential and selective. The fundamental idea behind this test is to identify organisms that use citrate as their only carbon source for metabolism, leading to alkalinity. The citrate is hydrolyzed by the citrate enzyme to produce acetic acid and oxaloacetic acid.
Composition of Simmons citrate (SIM) agar
- Dipotassium phosphate (1g/l)
- Sodium Citrate (2g/l)
- NaCl (5g/l)
- Agar (15g/l)
Materials
- Weighing balance
- Conical flask
- Measuring cylinder
- Filter paper
- Petri dish
- Aluminum foil paper
- Cotton plug
- Indicator tape
- Distilled water
- Hot plate
- Autoclave
Procedure
- Filter paper was placed on a Petri plate, which was then placed on a weighing scale and set to zero.
- 3 grams of Simmons Citrate Agar Base were weighed using a weighing balance.
- In a conical flask, suspend 3g of SIM agar base in 100ml of distilled water.
- Place a cotton plug on the flask, cover it with aluminum foil paper, and secure it with an indicator taper.
- The mixture should now be heated on a hot plate to eliminate any clumps.
- Next, autoclave the dissolved mixture for 15 minutes at 15 pounds of pressure and 121°C.
- The medium should be allowed to cool to room temperature after autoclaving.
- Fill the Petri plates with the medium once the temperature reaches 35 to 40°C.
- In order to evaporate the moisture, the plates were partially covered with a cap.
- It is recommended that solidified medium plates be incubated for 24 hours before being refrigerated.
- Triple Sugar Iron (TSI) agar
Principle
A differential medium is Triple Sugar Iron (TSI). Based on variations in the fermentation of carbohydrates and the formation of hydrogen sulfide, this agar distinguishes between species. When carbohydrates ferment, gas is produced and the color changes from red to yellow.
Composition of Triple Sugar Iron (TSI) agar
- Peptone (5g/l)
- Beef extract / yeast extract (3g/l)
- NaCl (0.5%)
- Agar (15g/l)
Materials
- Weighing balance
- Conical flask
- Measuring cylinder
- Filter paper
- Test tube
- Aluminum foil paper
- Cotton plug
- Indicator tape
- Distilled water
- Hot plate
- Autoclave
Procedure
- Filter paper was placed on a Petri plate, which was then placed on a weighing scale and set to zero.
- 5 grams of Simmons Citrate Agar Base were weighed using a weighing balance.
- In a conical flask, suspend 5g of TSI agar base in 100ml of distilled water.
- Place a cotton plug on the flask, cover it with aluminum foil paper, and secure it with an indicator taper.
- The mixture should now be heated on a hot plate to eliminate any clumps.
- Next, autoclave the dissolved mixture for 15 minutes at 15 pounds of pressure and 121°C.
- The medium should be allowed to cool to room temperature after autoclaving.
- Fill the test tubes with the medium once the temperature reaches 35 to 40°C.
- Tilt the test tubes slightly to release the moisture.
- It is recommended that solidified media plates be incubated for 24 hours before being refrigerated.